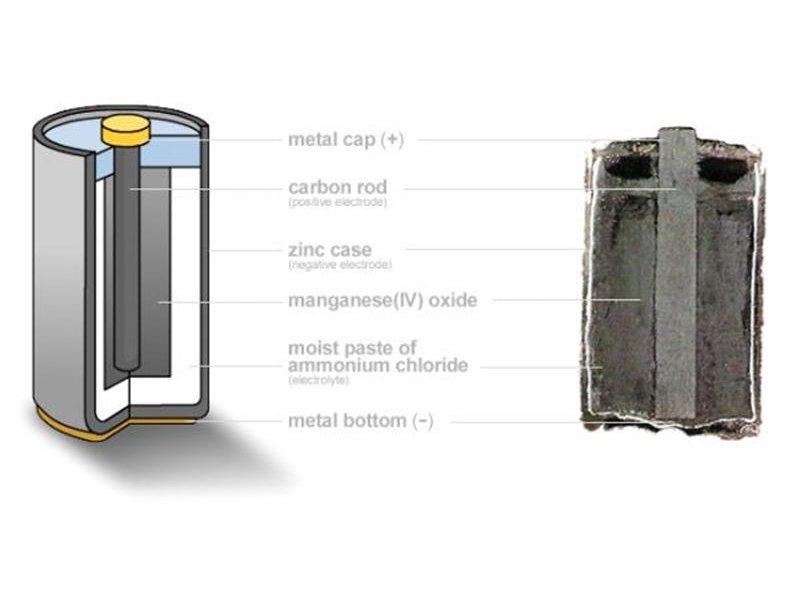
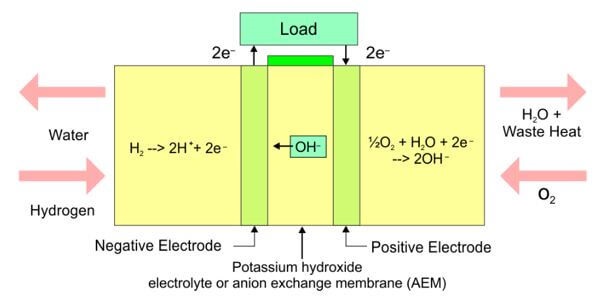
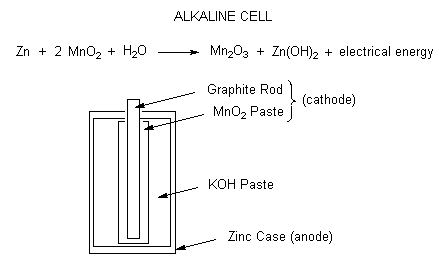
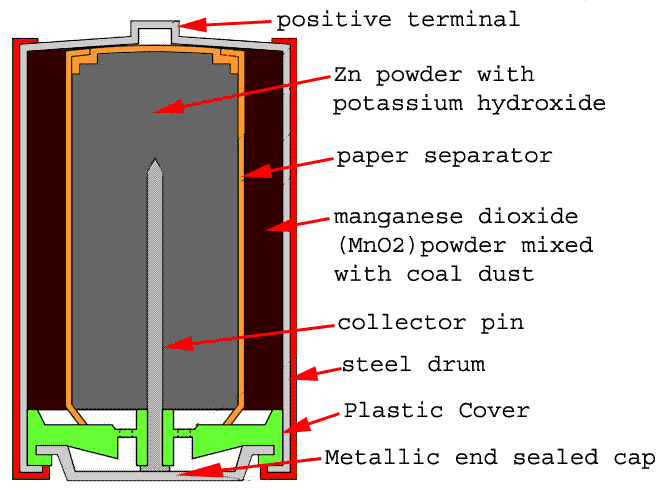
Schematic diagram of an alkaline Zn-MnO 2 battery showing electrode... | Download Scientific Diagram
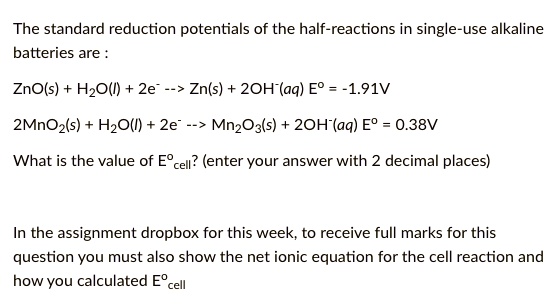
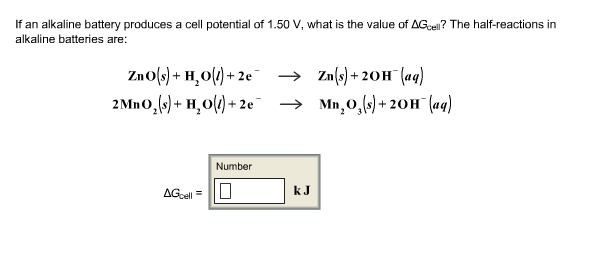
SOLVED: The standard reduction potentials of the half-reactions in single-use alkaline batteries are ZnO(s) Hzo() 2e Zn(s) 20H-(aq) Eo = -1.91V 2MnOz(s) HzO() 2e" MnzOz(s) 20H-(aq) Eo 0.38V What is the value


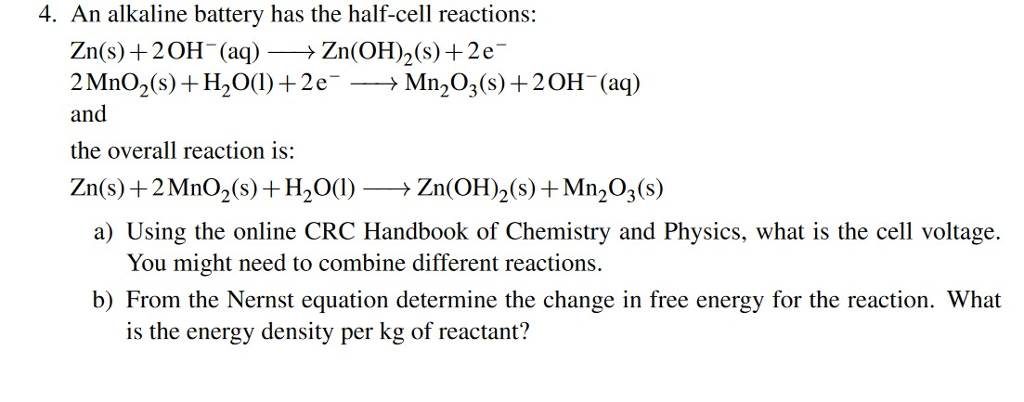
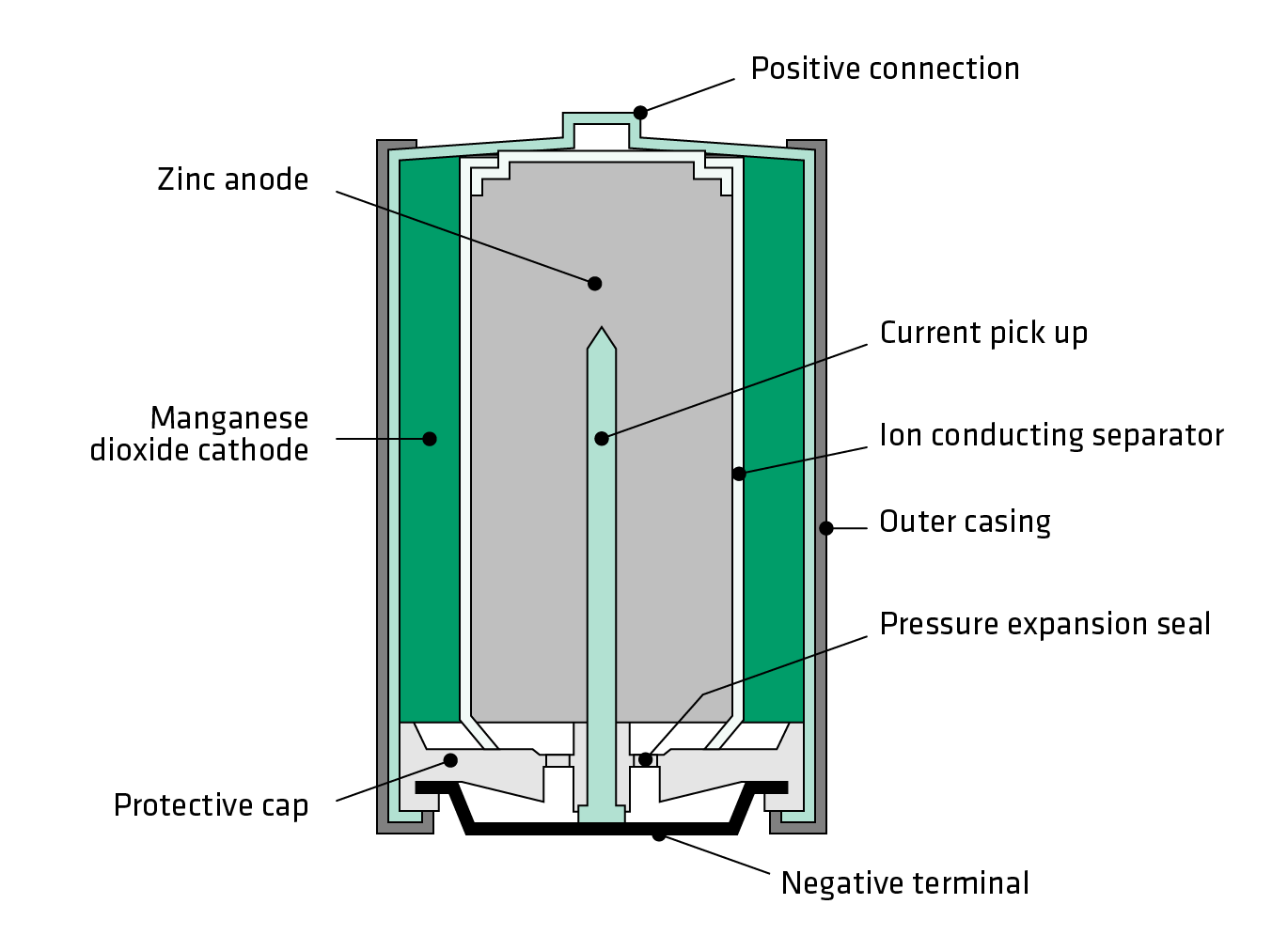





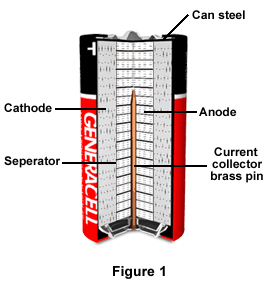

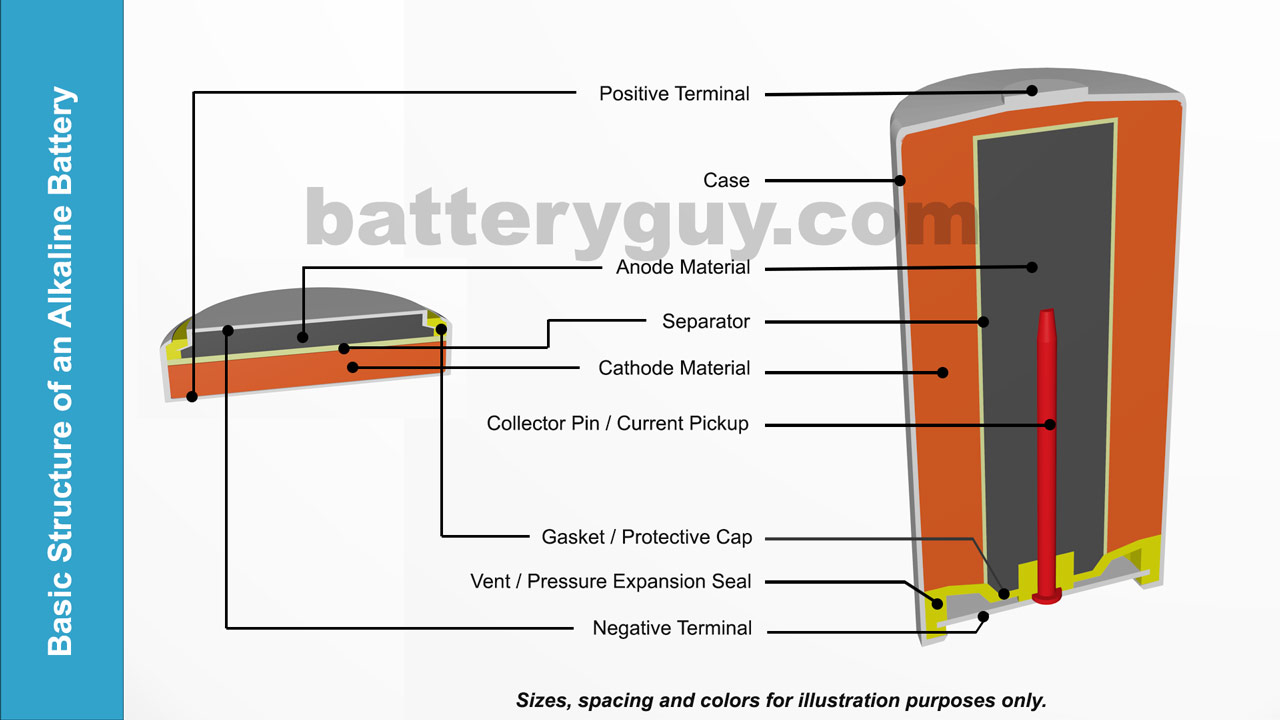

:format(jpeg)/www.theglobeandmail.com/ece-images/17d/incoming/article33465454.ece/BINARY/image.jpg)