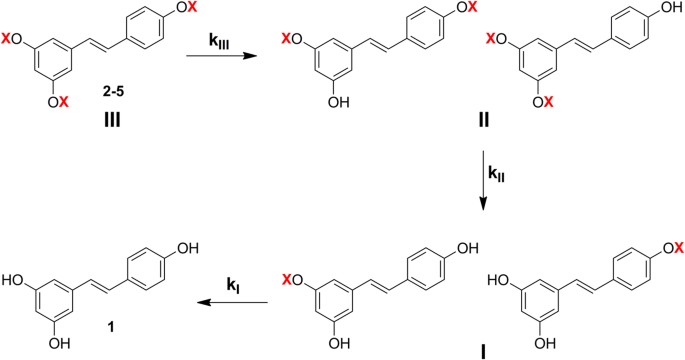
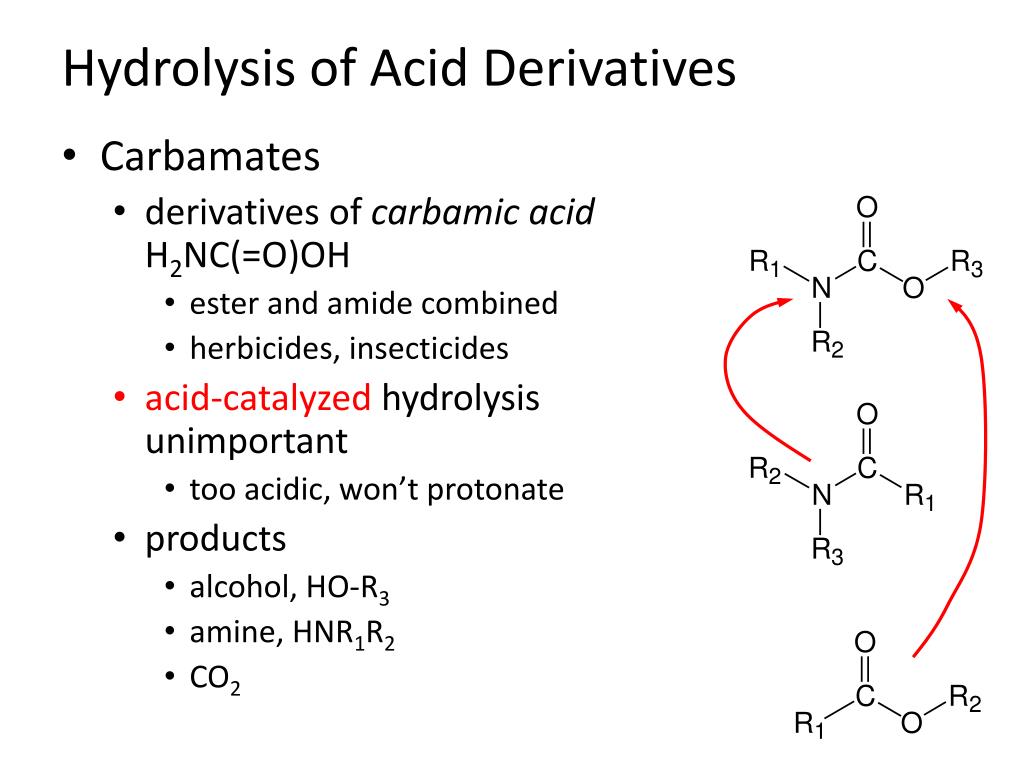
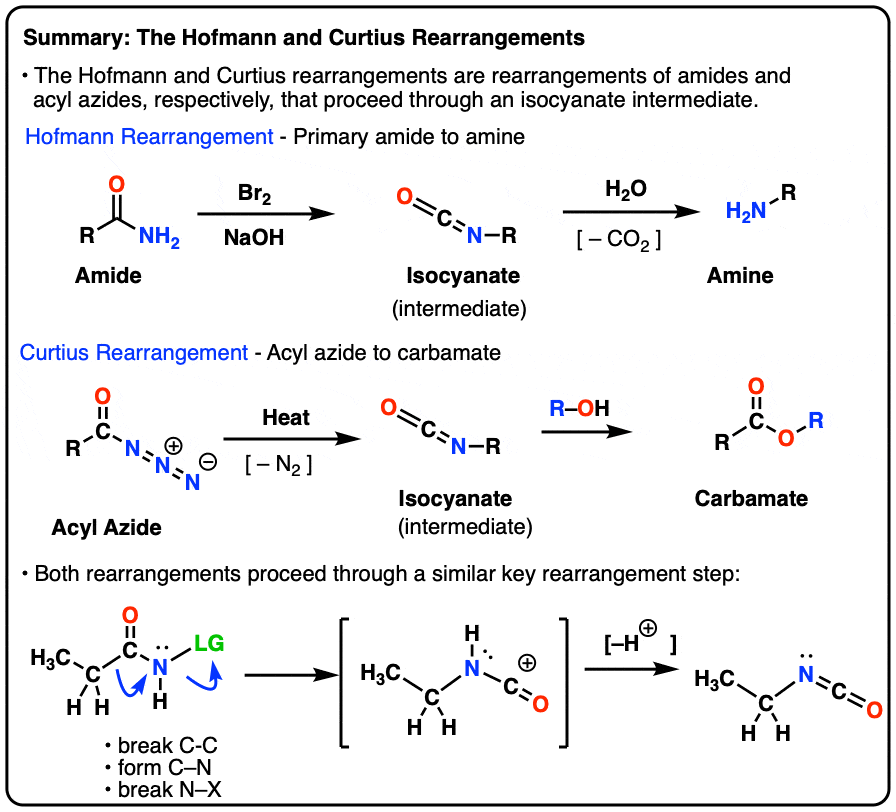
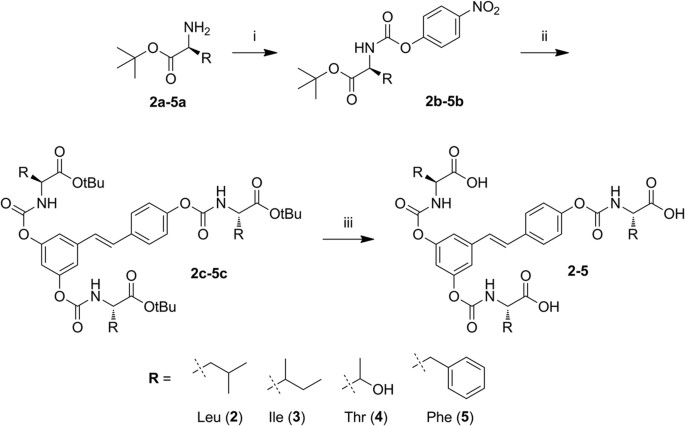
Antibody Catalysis of BAc2 Aryl Carbamate Ester Hydrolysis: A Highly Disfavored Chemical Process | Journal of the American Chemical Society

Hammett plot for hydrolysis of N -aryl pyridylcarbamates showing the e... | Download Scientific Diagram
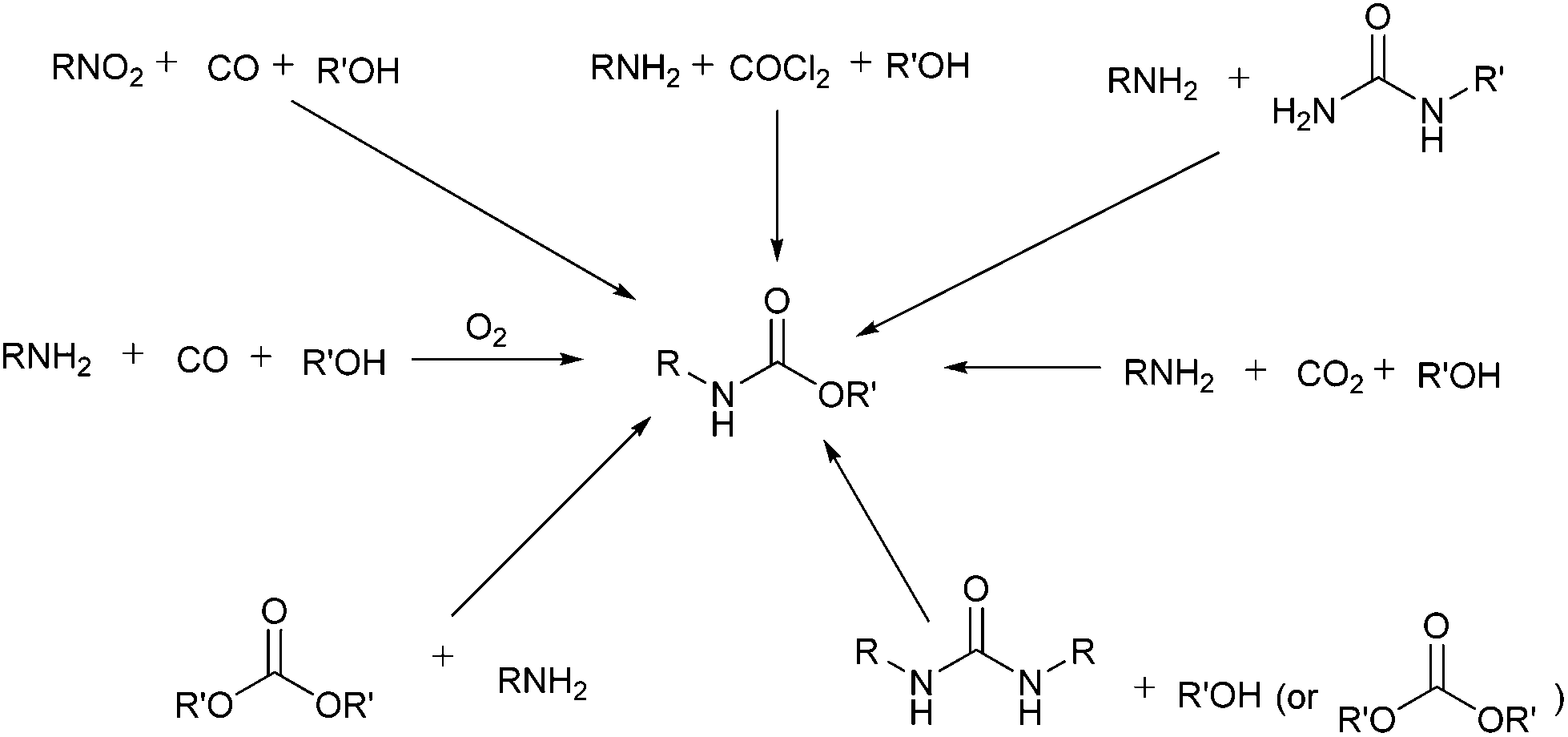
N -Substituted carbamate synthesis using urea as carbonyl source over TiO 2 –Cr 2 O 3 /SiO 2 catalyst - Green Chemistry (RSC Publishing) DOI:10.1039/C5GC01007A
Relative Stability of Formamidine and Carbamate Groups in the Bifunctional Pesticide Formetanate Hydrochloride

Mechanism of hydantoinase-catalyzed reaction. a Resonance forms for... | Download Scientific Diagram

Triazene drug metabolites. Part 17: synthesis and plasma hydrolysis of acyloxymethyl carbamate derivatives of antitumour triazenes - ScienceDirect
Carbamate group as structural motif in drugs: a review of carbamate derivatives used as therapeutic agents

Alkaline hydrolysis of tertiary N-(2-pyridyl)carbamates. Contradictory evidence between nucleophilic and general base catalysis | SpringerLink
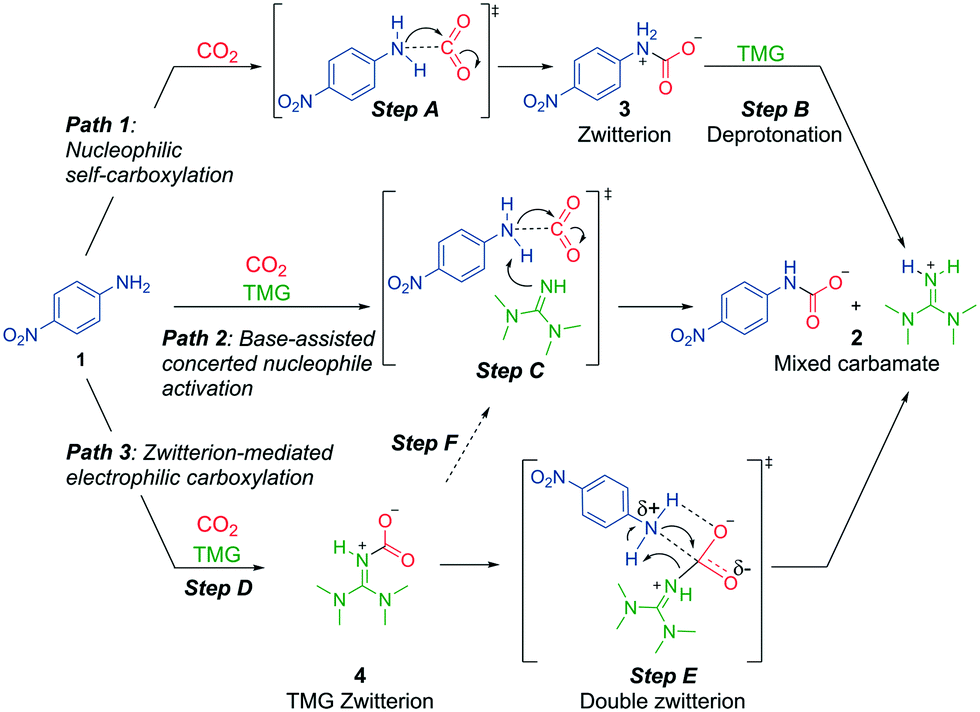
Mechanistic insights into carbamate formation from CO 2 and amines: the role of guanidine–CO 2 adducts - Catalysis Science & Technology (RSC Publishing) DOI:10.1039/D1CY01433A

Hydrolysis mechanism of esterases and amidases toward carbamate pesticides | Download Scientific Diagram
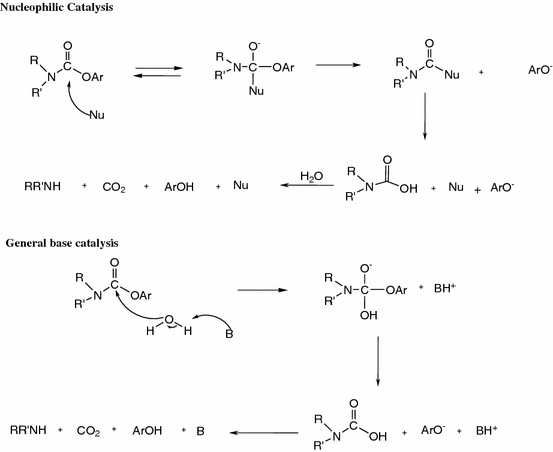
Alkaline hydrolysis of tertiary N-(2-pyridyl)carbamates. Contradictory evidence between nucleophilic and general base catalysis | SpringerLink

Hydrolysis is the most commonly encountered drug degradation mechanism, both in solution and in the solid state. Use the structure of ethyl ethanoate below to illustrate the mechanism of acid-catalyzed hydrolysis of